0.2 Five subcategories of Autotransporter proteins . The unfolded
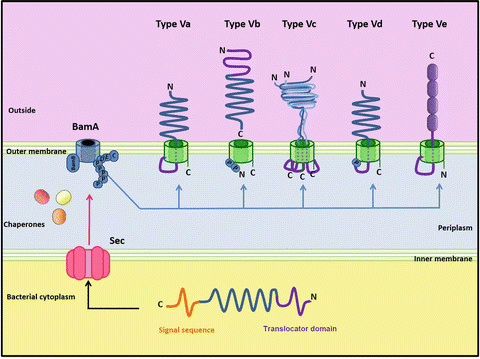
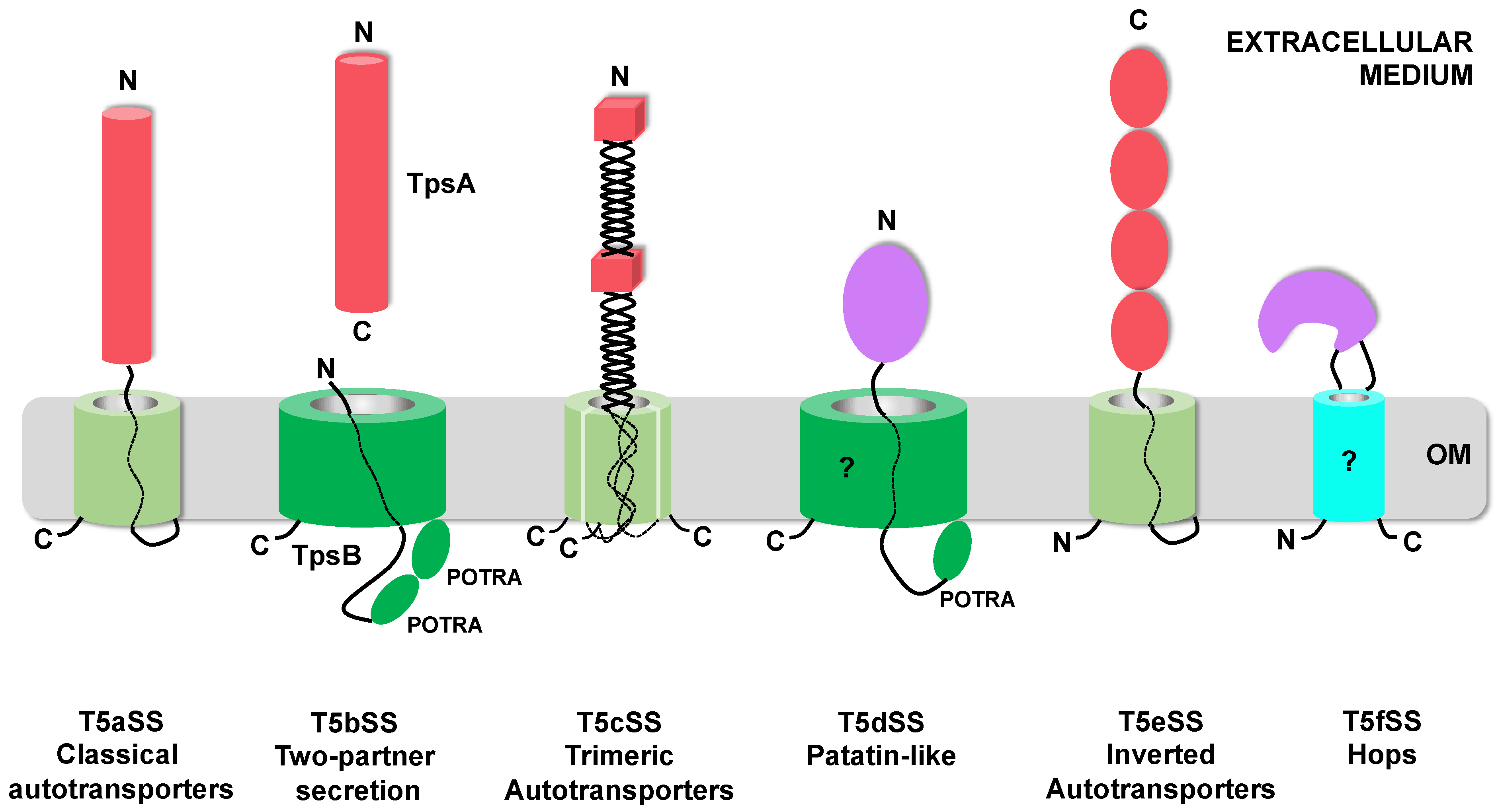
Download scientific diagram | 0.2 Five subcategories of Autotransporter proteins . The unfolded autotransporter of the T5SS is transferred to the periplasm by the SecYEG translocon, where several chaperones stabilize its unfolded structure. In the next stage, the translocator domain is inserted into the OM with the assistance of BamA, where it folds into a β-barrel. The unfolded passenger domain passes through the pore created by the translocator domain and folds into a β-helical structure. Structures and topology of the different type V secretion systems. The translocation domain is displayed in green, linker/Tps regions in purple, and passenger domains in blue. POTRA domains are labelled (P). The Type Va autotransporter model. The C-terminal membrane anchor (green) is recognized by the POTRA (P) domains of BamA; the Bam complex aids in inserting the β-barrel membrane anchor into the outer membrane (OM). The linker region (purple) then forms a hairpin inside the pore of the barrel. The passenger domain (blue) is pulled through the pore. The energy for this presumably derives from the sequential folding of the passenger domain on the outside of the cell. Once the passenger domain has been secreted, the linker assumes an α-helical conformation and plugs the pore. Type Vb autotransporter model. Vb subcategory are members of the two-partner secretion system (TPS) in which the passenger and the translocator domain harboring polypeptide-transport-associated (POTRA) motifs. The translocation proteins are known as TpsA and TpsB (green), and most of them are encoded in an operon. After their translocation through the inner membrane, TpsB forms the translocation β-barrel, which mediates secretion of the passenger protein (TpsA) through the outer membrane. Type Vc autotransporter model. Characterized by the presence of three passenger domains fused to the one-third segment of a fully functional C-terminal translocator domain allowing secretion of trimeric polypeptides. The Bam complex is required for trimeric autotransporter biogenesis and recognizes the C-terminal membrane anchor (purple). The Bam complex assists in trimerization of the β-barrel and membrane insertion. The linker regions (blue) form hairpins within the pore, and this leads to translocation of the polypeptides encoding the passenger domain. Type Vd autotransporter model. Autotransporters in this subcategory resemble a fusion between the Va, monomeric ATs, and the Vb, two partner system autotransporters. The passenger domain of PlpD (lipolytic enzyme) is connected to the translocator β-barrel with a POTRA domain and then is released by an autocatalytic reaction after translocation. Type Ve autotransporter model. Ve has an inverted topology with the passenger domain located in the C-terminal and the translocation unit in the N-terminal of the molecule; hence the term inverse autotransporter. Likewise, classical ATs, Ve are transported into the periplasm by the Sec translocon, assisted through the periplasm by common chaperones like SurA, and lastly, inserted into the outer membrane via the BAM complex from publication: Secretion Systems of Pathogenic Escherichia coli | Protein secretion plays a central role in modulating the interactions of bacteria with their environments. Bacterial ribosomes synthesize up to 8000 different proteins. Almost half of these become integrated in membranes and are secreted to the periplasm or to the external | Protein Secretion, Secretion and Bodily Secretions | ResearchGate, the professional network for scientists.

Bacterial Secretion Systems for Use in Biotechnology: Autotransporter-Based Cell Surface Display and Ultrahigh-Throughput Screening of Large Protein Libraries
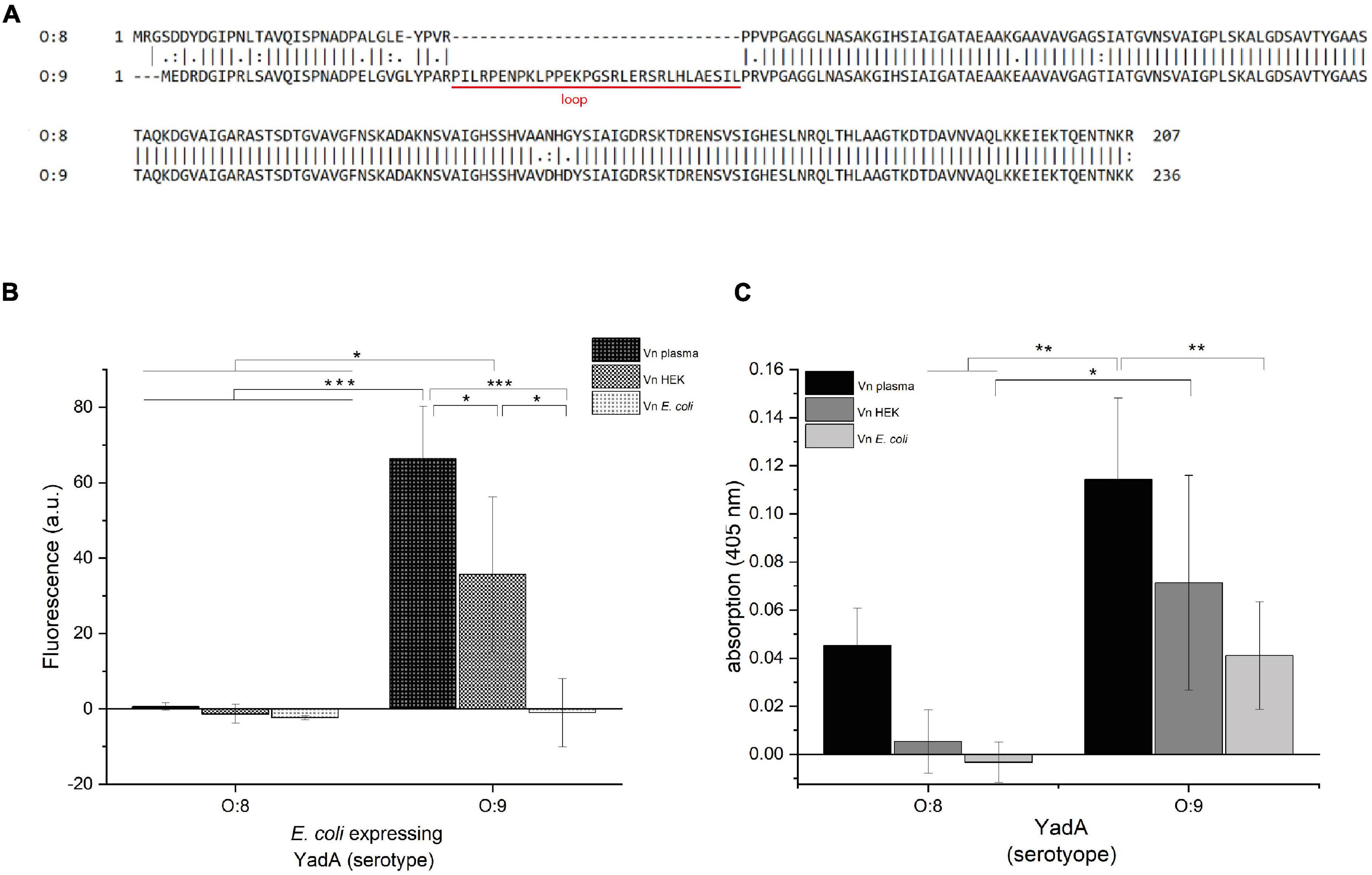
Frontiers The Trimeric Autotransporter Adhesin YadA of Yersinia enterocolitica Serotype O:9 Binds Glycan Moieties
Toxins, Free Full-Text
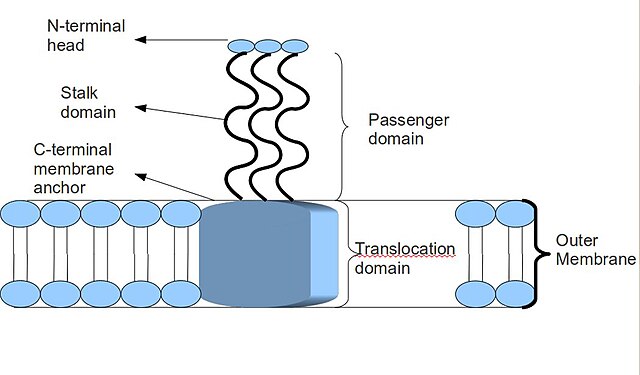
Trimeric autotransporter adhesin - Wikipedia

Protein SecB - an overview

Type V Protein Secretion Pathway: the Autotransporter Story

Unmasking of the von Willebrand A-domain surface adhesin CglB at bacterial focal adhesions mediates myxobacterial gliding motility

Flexible client-dependent cages in the assembly landscape of the periplasmic protease-chaperone DegP

Fine tuning the mechanism of Antigen 43 self-association to modulate aggregation levels of Escherichia coli pathogens

BamA and BamD are essential for the secretion of trimeric autotransporter adhesins

The Escherichia coli outer membrane protein OmpA acquires secondary structure prior to its integration into the membrane - ScienceDirect

Exploiting pglB Oligosaccharyltransferase-Positive and -Negative Campylobacter jejuni and a Multiprotease Digestion Strategy to Identify Novel Sites Modified by N-Linked Protein Glycosylation

Mitochondria can recognize and assemble fragments of a β-barrel structure