Solved overall reaction: 2NO(g)+O2(g)→2NO2(g) Step


29 For reaction, 2NO(g)+O2( g)⟶2NO2( g) Rate law is : rate of reaction ..
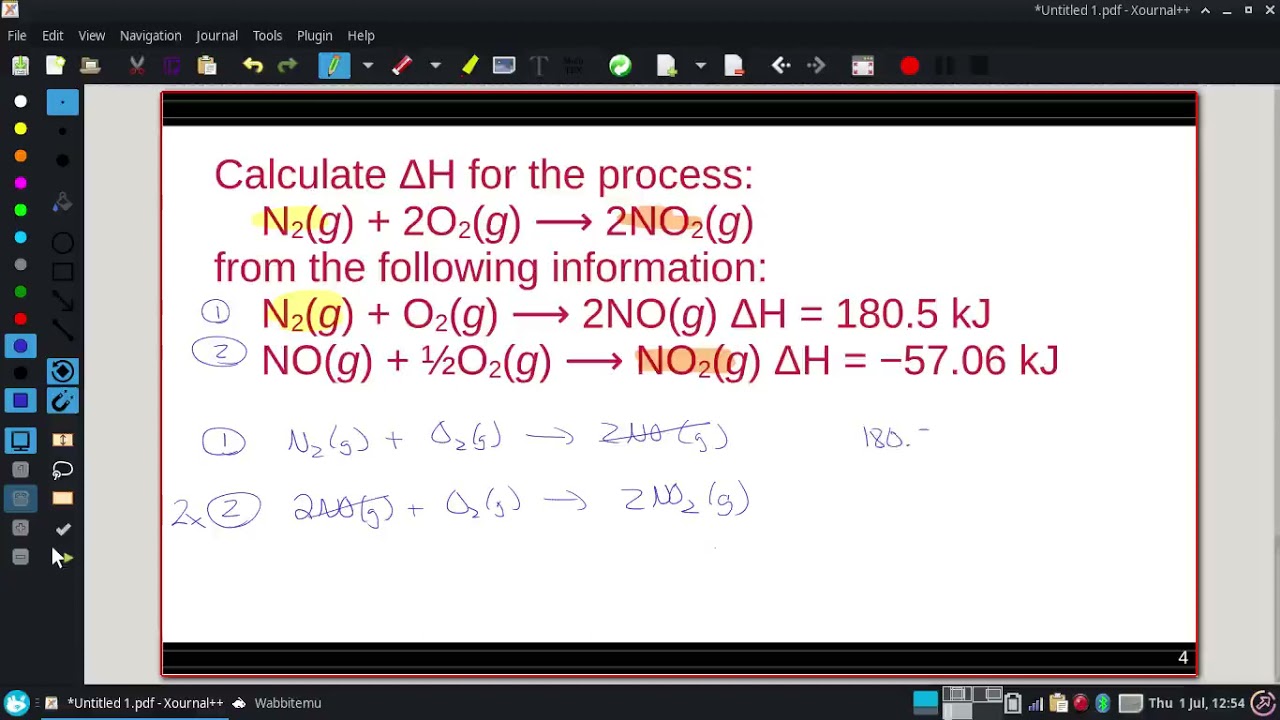
Calculate ΔH for the process: N2(g) + 2O2(g) ⟶ 2NO2(g)
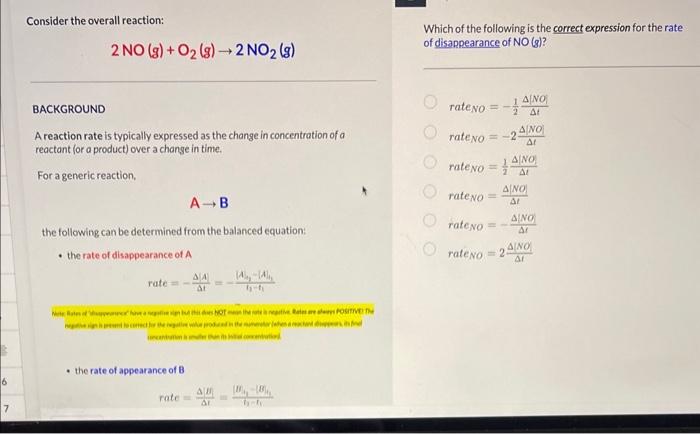
Solved Consider the overall reaction: 2NO(g)+O2(g)→2NO2(g)

Answered: 6.1 Calculate the enthalpy of the…

SOLVED: The following two-step mechanism has been proposed: Overall reaction: 2NO(g) + O2(g) -> 2NO2(g) Step 1: NO + O2 -> NO3 Step 2: NO3 + NO -> 2NO2 slow fast What

4. The dissociation of NO2 occurs by the following reaction: 2NO2(g) → 2NO(g) + O2(g) If the rate for the

The reaction 2NO + Br_{2} rightarrow 2NOBr follows the mechanism:(1) NO + Br_{2} overset{Fast}{rightleftharpoons} NOBr_2(2) NOBr_2 + NO xrightarrow{Slow} 2NOBrWhich of the following is/are true regarding this?The order of the reaction with

For the reaction 2NO(g)+O2( g)→2NO2( g) calculate ΔG at 700 K when enth..
53. Show by using rate law how much rate of rhe reaction, 2NO + O2 > 2NO2 will change if the volume of the reaction vessel is reduced to 1/3rd of its initial value.
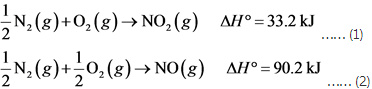
Calculate the enthalpy of the reaction 2NO(g)+O2(g)→2NO2(g) - Home Work Help - Learn CBSE Forum

2NO(g)+O2(g)→2NO2(g) r=K[NO]2[O2

consider the following reaction 2no(g)+o2(g)→2no2(g). calculate ∆g° at 298k and predict weather the reaction