a)Adsorption energy comparison between twice of * NO and N-N

Download scientific diagram | (a)Adsorption energy comparison between twice of * NO and N-N coupling intermediate * ONNO. The horizontal line shows the equilibrium between 2NO(g) and 2 * NO, while the diagonal line illustrates the equal adsorption energy between 2 * NO and * ONNO. (b)The activation barriers for N-N coupling between * N and NO (gas) vs * N and * N. where the unfilled markers show around 0.44 Monolayer of NO coverage is considered. (c) Adsorption energy comparison between * NO and * N 2 O.The horizontal line shows the equilibrium between NO(g) and * NO while the vertical line depicts the equilibrium between N 2 O and * N 2 O under standard conditions. from publication: Electrochemical Nitric Oxide Reduction on Metal Surfaces | Electrocatalytic denitrification is a promising technology for removing NOx species (NO3− , NO2− and NO). For NOx electroreduction (NOxRR), there is a desire for understanding the catalytic parameters that control the product distribution. Here, we elucidate selectivity and | Nitric Oxide, Electrochemistry and CO2 Reduction | ResearchGate, the professional network for scientists.
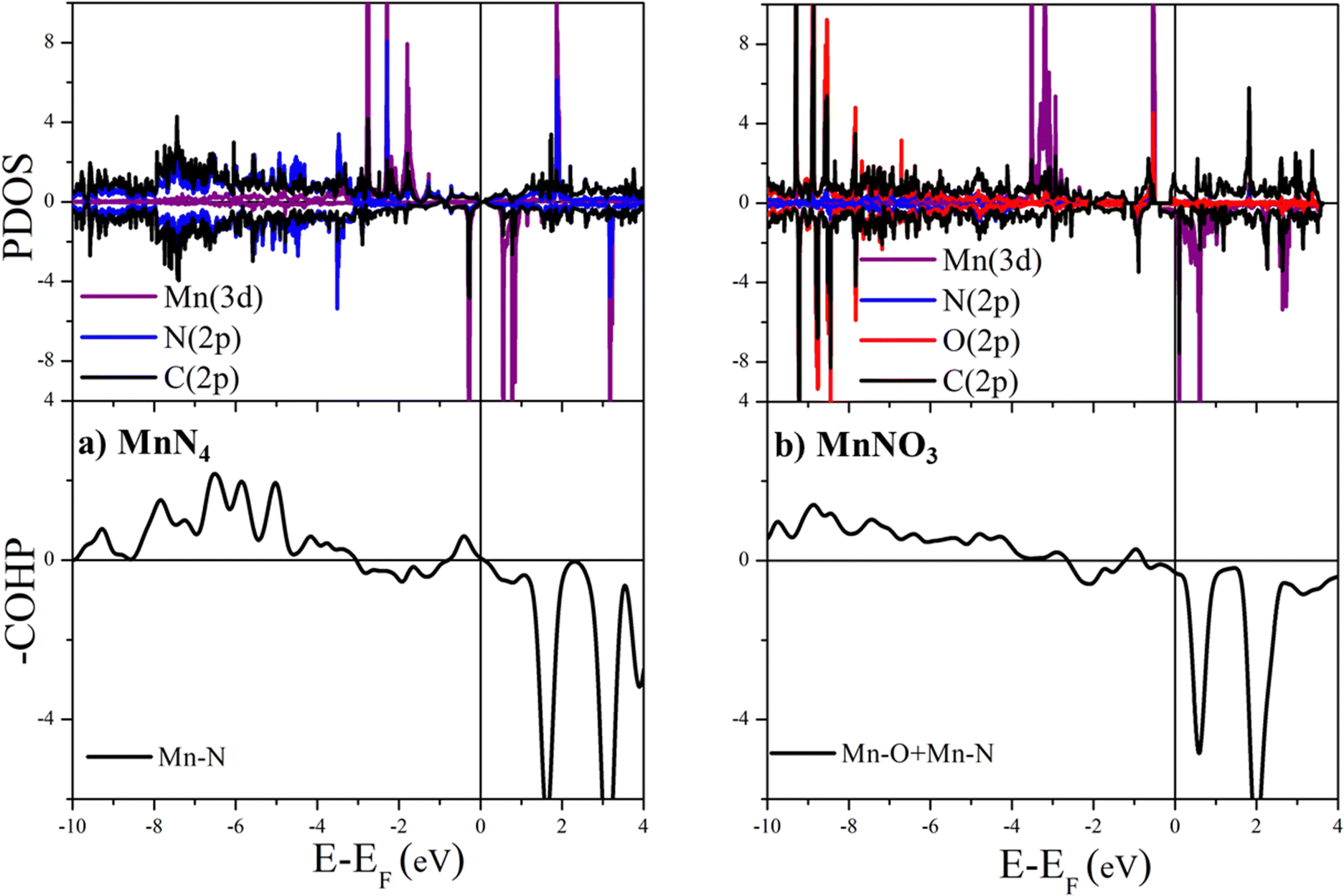
Adsorption mechanism of the N 2 and NRR intermediates on oxygen modified MnN 4 –graphene layers – a single atom catalysis perspective - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP05491D

Density functional theory study of the adsorption of NO on CunX (n = 2–8; X = Cu, K) clusters - Cheng - 2024 - International Journal of Quantum Chemistry - Wiley Online Library

Average adsorption energies versus the number of adsorbed CO 2

A comparative density functional theory study of oxygen doping versus adsorption on graphene to tune its band gap - ScienceDirect

a)Adsorption energy comparison between twice of * NO and N-N coupling
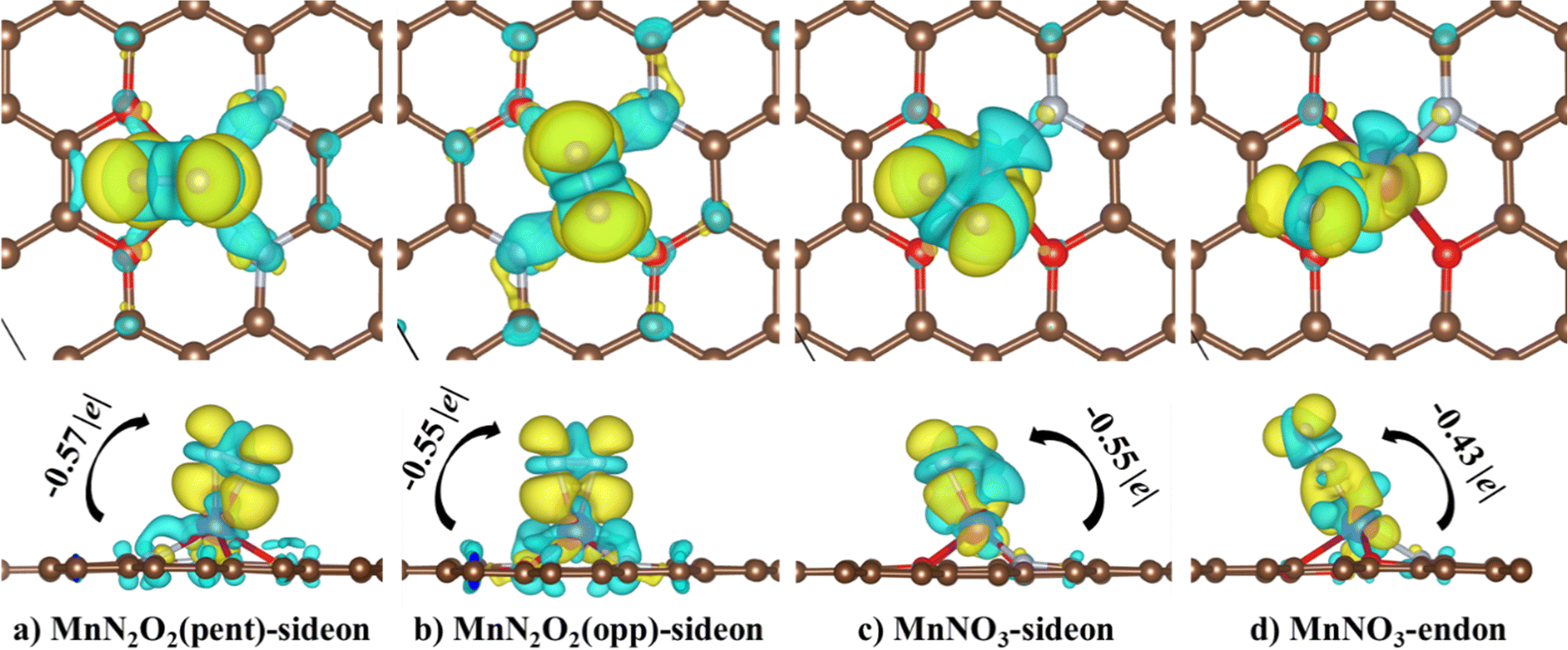
Adsorption mechanism of the N 2 and NRR intermediates on oxygen modified MnN 4 –graphene layers – a single atom catalysis perspective - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP05491D

The bond length, adsorption energy and energy barrier of the ethanol

Average adsorption energies versus the number of adsorbed CO 2

Adsorption and migration behaviours of Nb–C atoms on clean diamond (0 0 1) surface: A first principles study - ScienceDirect

Adsorption configurations of NO, NO2 and N2O onto intrinsic graphene