Making buffer solutions: Video, Anatomy & Definition


Using the Henderson-Hasselbalch Equation for a Buffer, Chemistry

SOLVED: One of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into
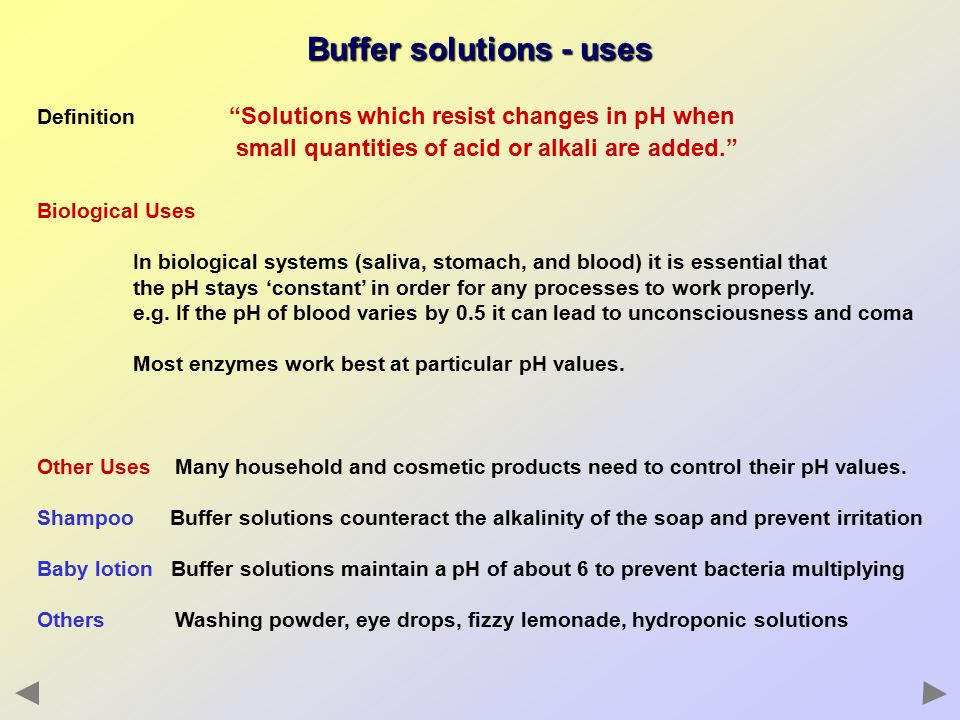
BUFFER SOLUTIONS. - ppt download
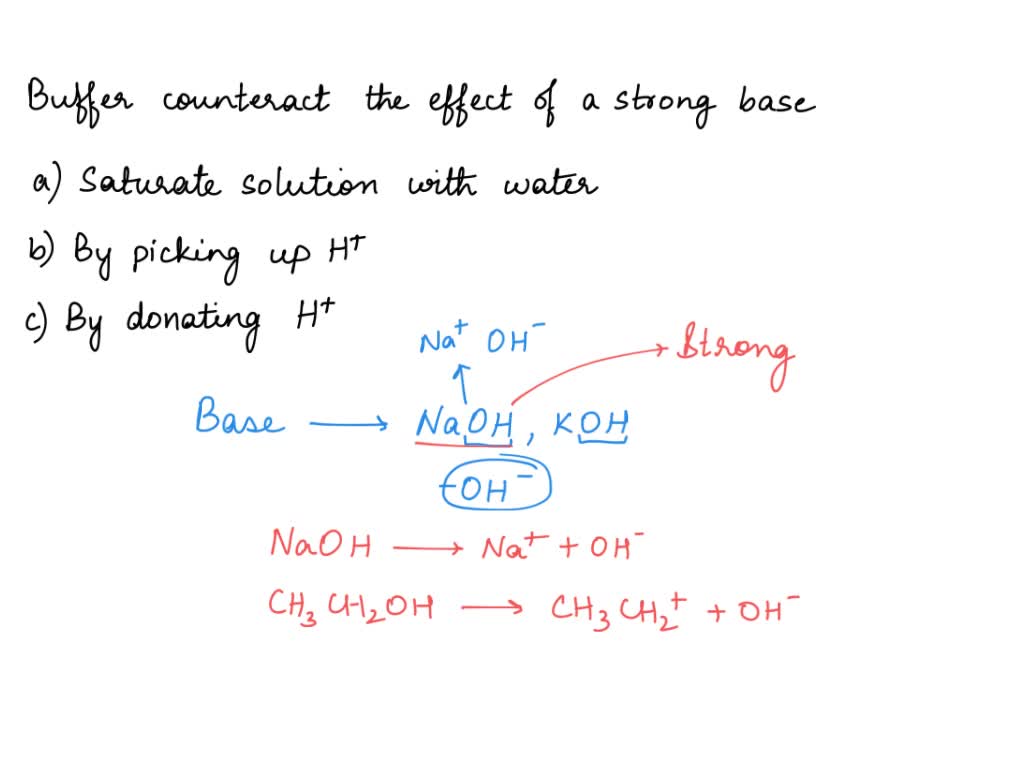
SOLVED: How does a buffer counteract the effect of a strong base? Select one: A. By saturating the solution with water B. By picking up H+ C. By donating H+

Buffer System in Chemistry, Definition, Function & Examples - Video & Lesson Transcript
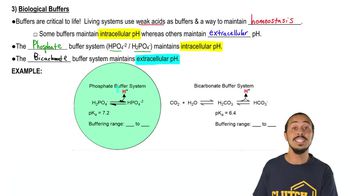
Buffer Solution Video Tutorial & Practice

Buffer Solutions Part-1, Definition

Assessing Cardiac Contractility From Single Molecules to Whole Hearts - ScienceDirect

Buffer Solutions

Buffer solutions Buffer solution, Chemistry lessons, Ap chemistry
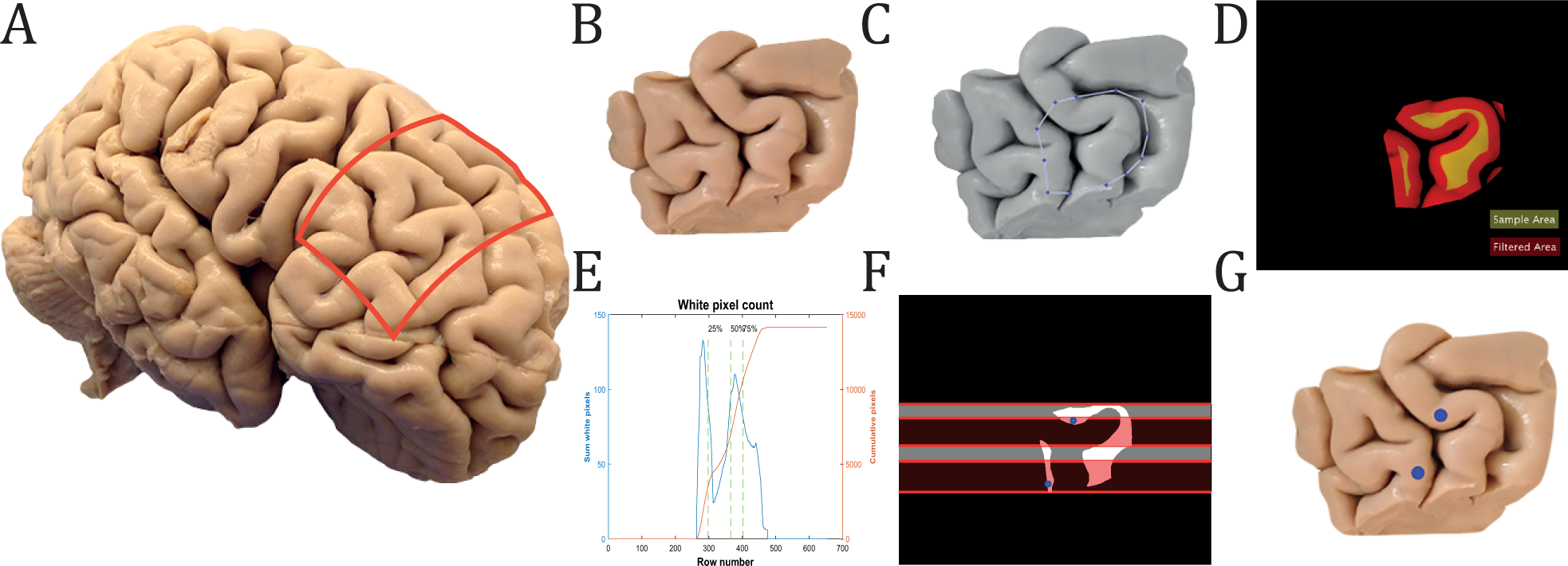
Cellular 3D-reconstruction and analysis in the human cerebral cortex using automatic serial sections

Molarity and dilutions: Video, Anatomy & Definition

Inner Ear Anatomy - Description and FAQs