FDA Outlines Generic Safety and Efficacy - Policy and Medicine

Saunders Nursing Drug Handbook 2024: 9780443116070: Medicine & Health Science Books @

A pragmatic regulatory approach for complex generics through the U.S. FDA 505(j) or 505(b)(2) approval pathways - Klein - 2021 - Annals of the New York Academy of Sciences - Wiley Online Library
Patient Labeling Resources

Scientific and regulatory activities initiated by the U.S. food and drug administration to foster approvals of generic dry powder inhalers: Quality perspective - ScienceDirect

Drug Safety Data Curation and Modeling in ChEMBL: Boxed Warnings and Withdrawn Drugs

Foods, Free Full-Text

FDA Solicits Feedback on the Use of AI and Machine Learning in Drug Development - Bill of Health

Understanding US Food and Drug Administration (FDA) Approval Processes

How FDA Approves Drugs and Regulates Their Safety and Effectiveness

FDA on products intended for general wellness

Safety, Efficacy, and Quality Remain Top Priorities as We Continue Our Work to Expand Access to Cost-Saving Generic Drugs for the American Public
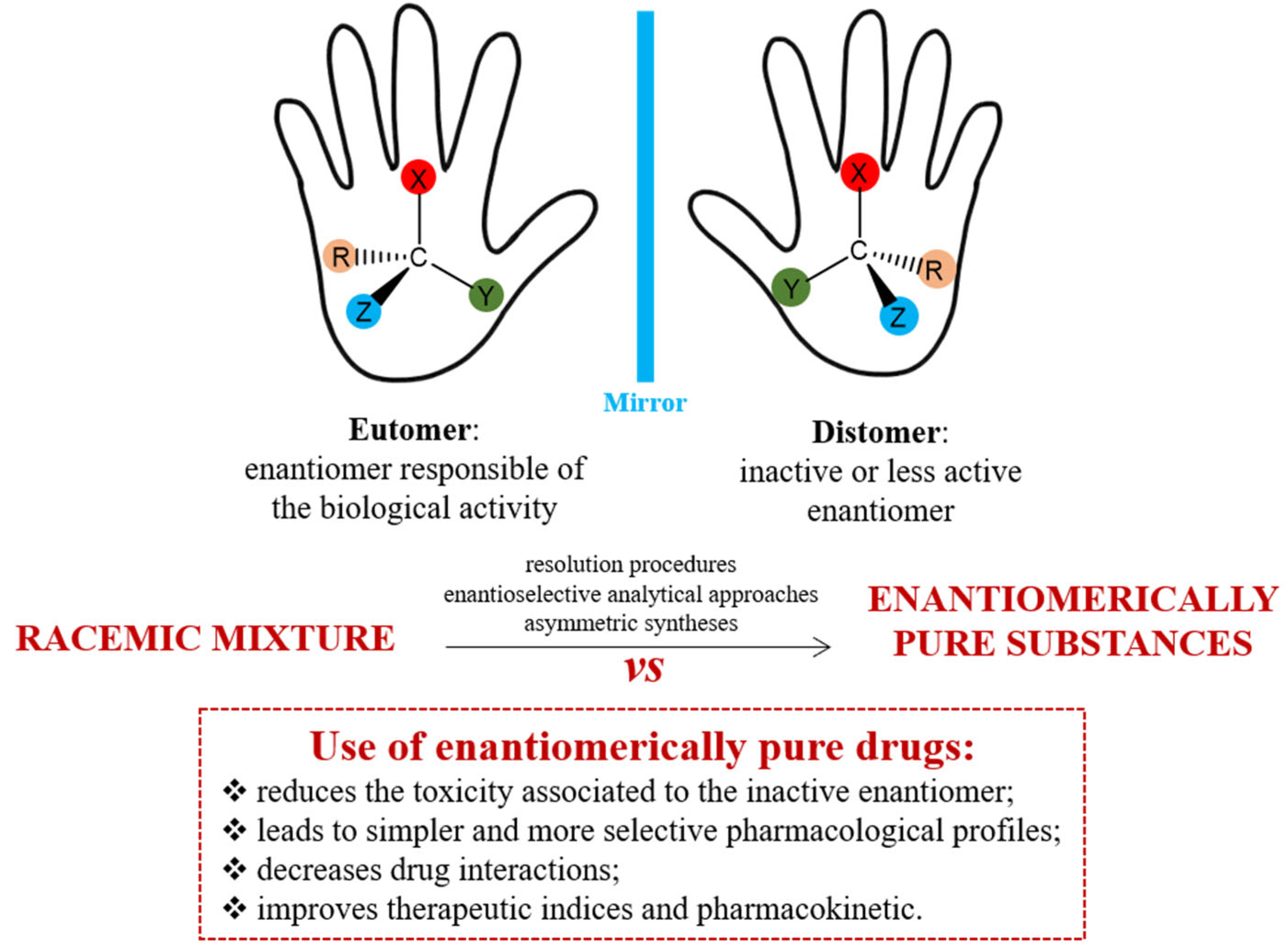
Applied Sciences, Free Full-Text

2020 at FDA: A Year of Unparalleled Contributions to Public Health

Regulating and Authorizing Medicines: A Comparison of the FDA and EMA