Membrane binding of pore-forming γ-hemolysin components studied at different lipid compositions - ScienceDirect


Cryo-EM-based structural insights into supramolecular assemblies of γ- hemolysin from S. aureus reveal the pore formation mechanism - ScienceDirect
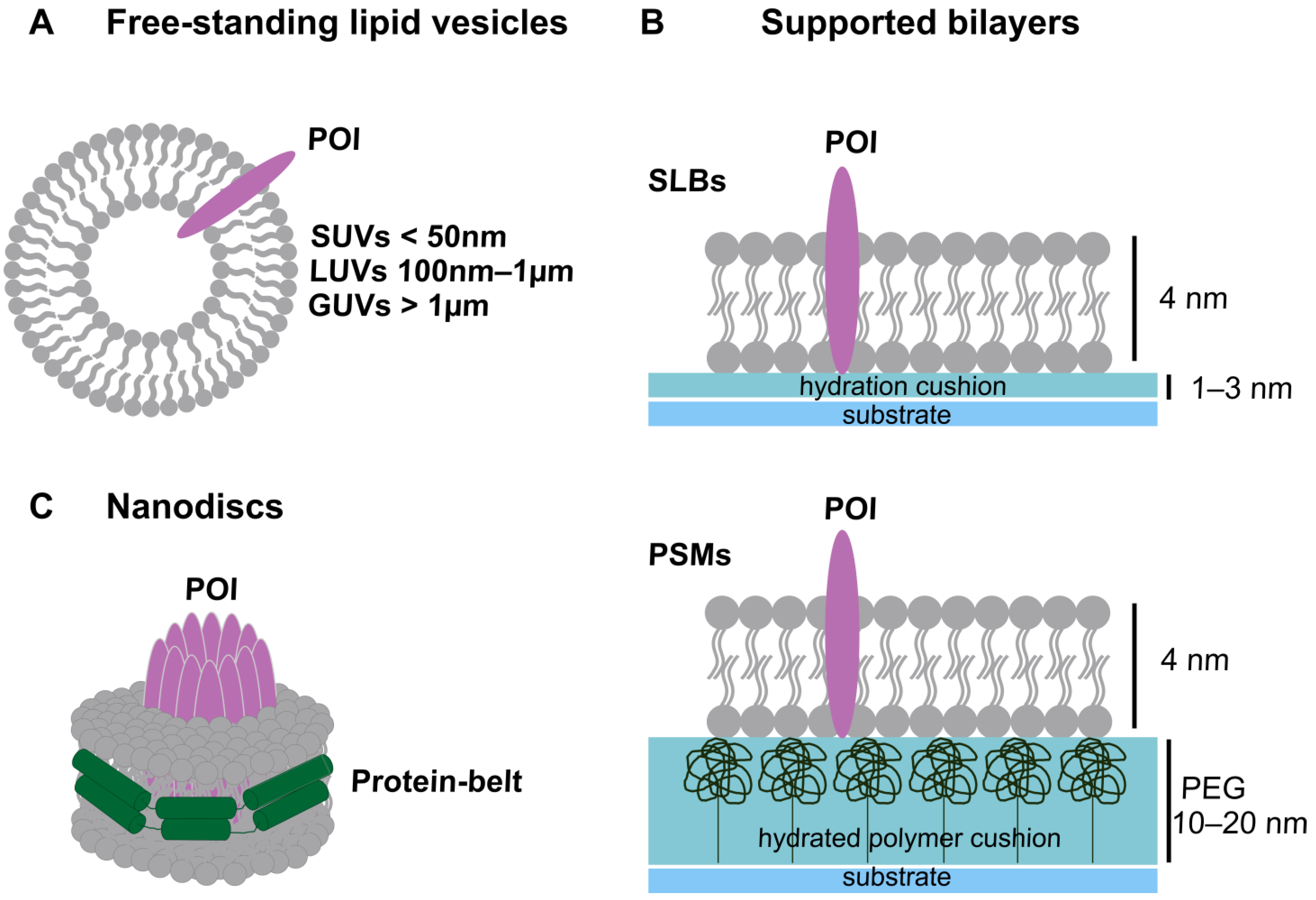
IJMS, Free Full-Text

γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM Investigation - ScienceDirect

Cell targeting by the Staphylococcus aureus pore-forming toxins: it's not just about lipids: Trends in Microbiology

γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM Investigation - ScienceDirect

Cryo-EM-based structural insights into supramolecular assemblies of γ- hemolysin from S. aureus reveal the pore formation mechanism - ScienceDirect

Membrane binding of pore-forming γ-hemolysin components studied at different lipid compositions - ScienceDirect

Protein–lipid interactions and non-lamellar lipidic structures in membrane pore formation and membrane fusion - ScienceDirect

γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM Investigation - ScienceDirect
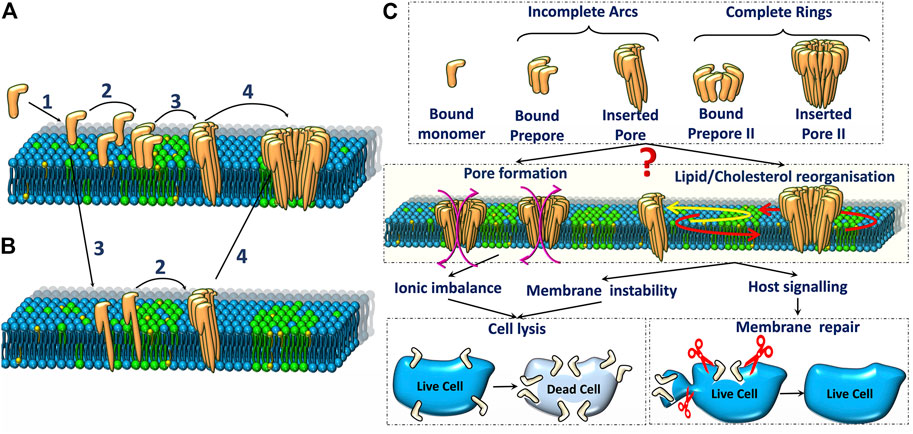
Frontiers Pore Forming Protein Induced Biomembrane Reorganization and Dynamics: A Focused Review
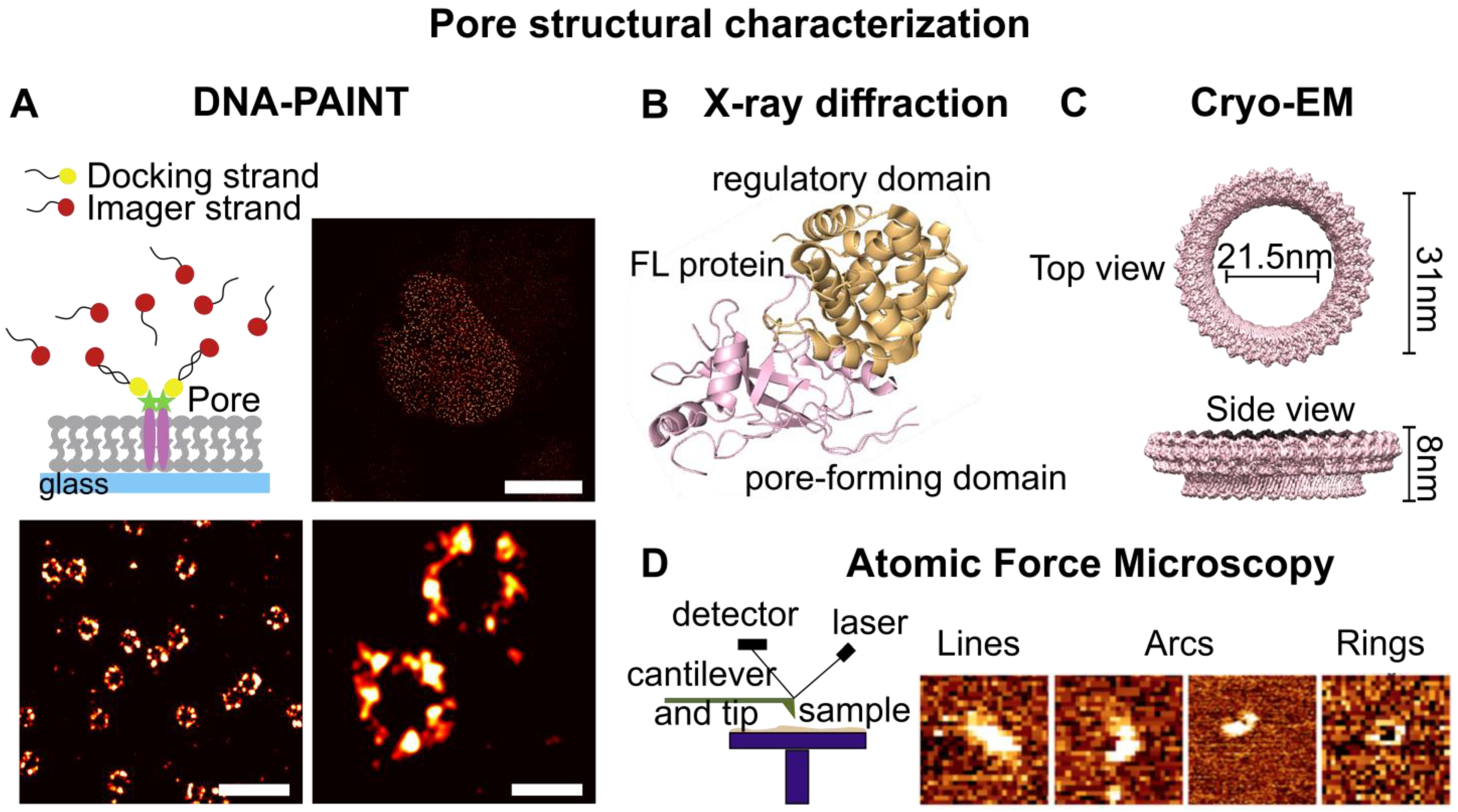
IJMS, Free Full-Text

Assembly of streptolysin O pores assessed by quartz crystal microbalance and atomic force microscopy provides evidence for the formation of anchored but incomplete oligomers - ScienceDirect

Membrane binding of pore-forming γ-hemolysin components studied at different lipid compositions

Assembling the puzzle: Oligomerization of α-pore forming proteins in membranes - ScienceDirect