BNT162b2 Vaccine Booster and Mortality Due to Covid-19

The safety profile of the first and second monovalent and bivalent BNT162b2 mRNA booster vaccines in older adults
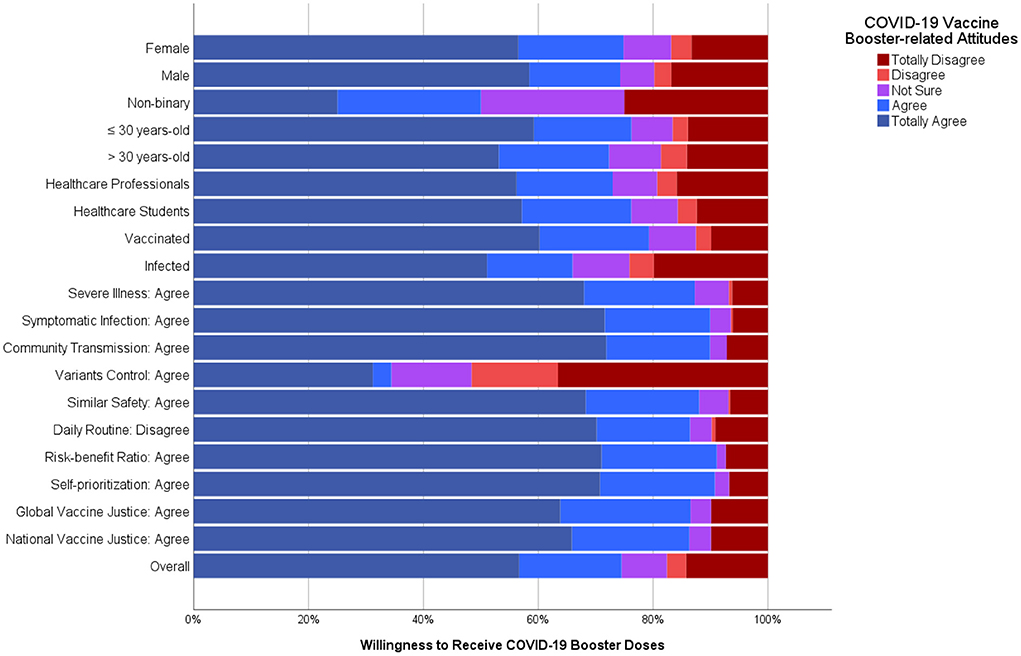
Frontiers COVID-19 vaccine booster hesitancy (VBH) of healthcare professionals and students in Poland: Cross-sectional survey-based study

Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years
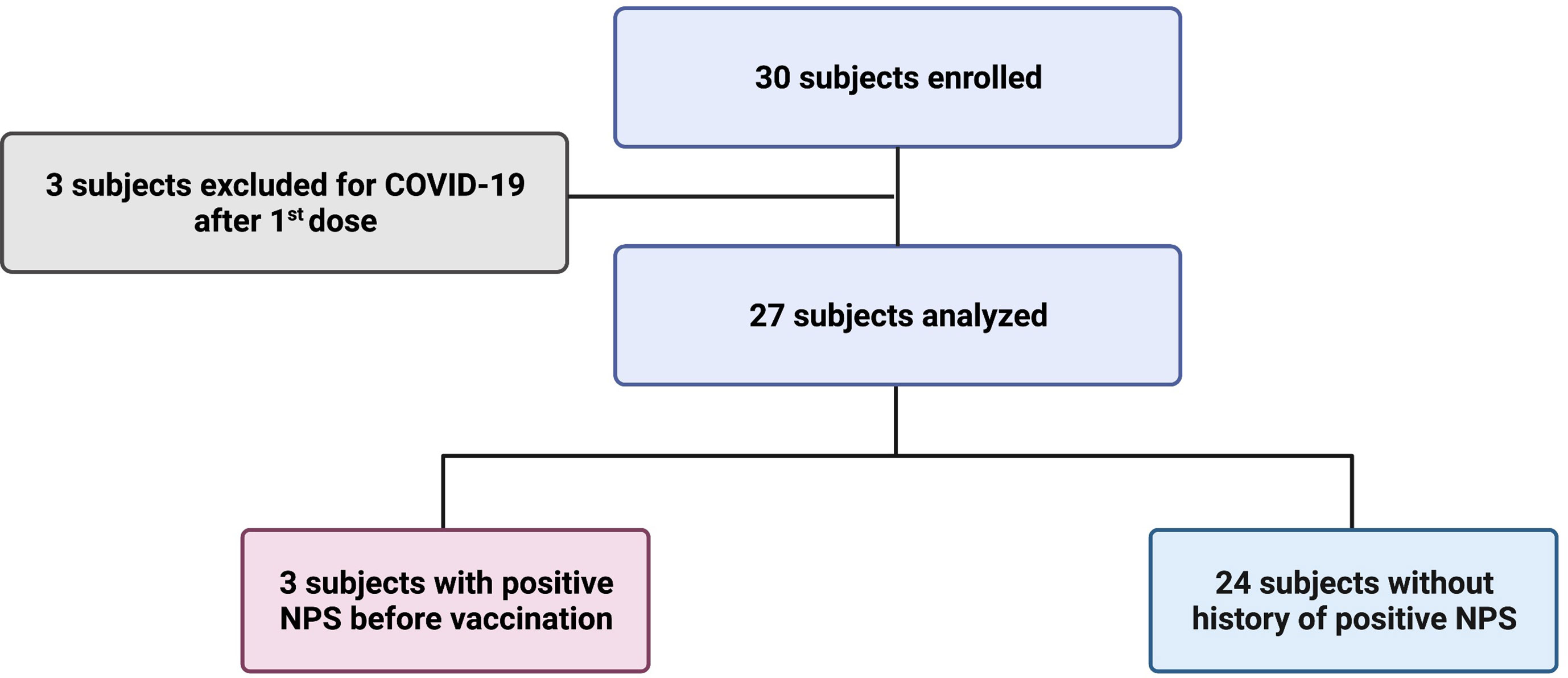
Frontiers The BNT162b2 vaccine induces humoral and cellular immune memory to SARS-CoV-2 Wuhan strain and the Omicron variant in children 5 to 11 years of age
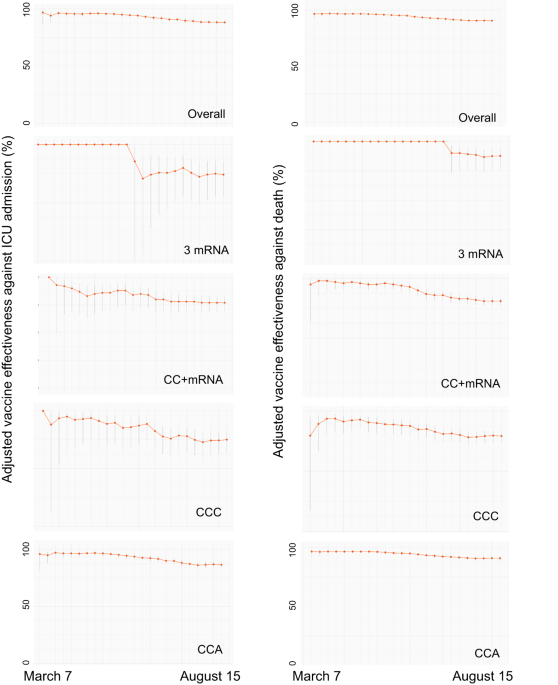
Effectiveness of the second COVID-19 booster against Omicron: a large-scale cohort study in Chile
Vaccines, Free Full-Text

Finally, Real-World Effectiveness Data on Pfizer Boosters Across Ages

Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine

Effectiveness of mRNA-1273, BNT162b2, and BBIBP-CorV vaccines against infection and mortality in children in Argentina, during predominance of delta and omicron covid-19 variants: test negative, case-control study

COVID-19 vaccines show efficacy for preventing hospitalization, death in patients with CKD

Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults — Increasing Community Access to Testing

Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: a test-negative case–control study - The Lancet Respiratory Medicine

Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study